WATER
What is WATER?
I remember during our class in Food Chemistry, our instructor asked us, "What is water?" I answered, "Water is the universal solvent," but my instructor made me realize that water is more than that; it plays a crucial role in the food industry.
Water is a substance that all living organisms, including microorganisms, need in order to survive. It has a composition of two hydrogen (H) atoms and one oxygen (O) atom, also known as H20. Some physical properties of water are tasteless, colorless, and odorless. It has a 100 degree Celsius high boiling point and a melting point of 0 degrees Celsius. Water density at 4.0 degrees Celsius is 1.0 g/mL and ice density at 0 degrees Celsius is 0.91 g/mL. It has a specific heat of 1.0 cal/g degree Celsius or 4.184 J/g degree Celsius. Water is also known for its unusual properties. It exists in all three states of matter; it can be solid, liquid, or gas. Another unusual property that makes me amused is that when water is in a solid state, it is less dense than liquid. As a result, ice floats on water because when water solidifies, most of its substance contracts. Furthermore, it has a polar molecule that is both positively (+) and negatively (-) charged that can bind to any substance like sugar and salt. That is why water is one of the vital elements in foods by knowing the water activity and moisture content.
What is moisture content?
Moisture content is one of the most significant indicators in processing and food testing. According to Fennema’s Food Chemistry Book, moisture content is a calculation of yield and solid food quantity that can be a firsthand index of economic value, stability, and quality of food products.
What is water activity?
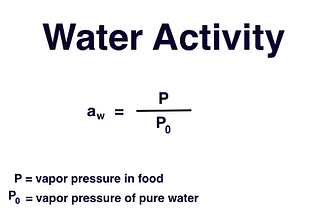
Food scientists use this term to describe the amount of water available to microbes, enzymes, and other reactions within food. It is one of the major factors among many that affect the stability of the food. In the laboratory, water activity is represented by the aw notation or expression as aw=P/Po, where P is the vapor pressure of water in the sample and Po is the vapor pressure of pure water.
How can I determine water activity in foods?
The water activity level of food is used to know if the food should be left behind or stay cold. Water activity starts from 0.0 aw, which is bone dry, to 1.0 aw, which is pure water. For example, we have raisins, crackers, peanut butter, strawberries, and pre-cooked hamburgers. Pre-cooked hamburgers have a 0.95 aw; strawberries have 0.92 aw; peanut butter has 0.70 aw, and crackers have 0.20 aw. Low water activity makes foods last longer, like crackers, because at .60 aw, most microbes stop growing, while high water activity makes foods easily spoil and must be refrigerated to prevent the multiplication of microorganisms. Therefore, foods with a higher than normal 0.85 aw are considered potentially hazardous foods (PHFs).
Why does some moist food not spoil quickly?
We may wonder how some moist foods that also have higher water activity, like jams, don't spoil quickly. Why? As mentioned above, water has polar properties. It can bind to another substance, so even though jams contain around 50–60% water, it is already attached to sugar molecules. As a result, water is not available for microbes to grow, which prevents spoilage, similarly to salting.
Is water activity and moisture content the same?
No, because water activity is related to solving issues associated with quality like microbial growth, sogginess, crispness, caking, staling, browning, enzymatic activity, and shelf life of food. While moisture content is to solve issues regarding quantity, it also includes nutrition information, potency, and yield, etc.
Why is the water activity important?
Understanding water activity as one of the major food components has served as a powerful weapon for preserving foods since then. Furthermore, it inspired us to create food packaging as well as safe and long-lasting food products.






Comments
Post a Comment